Introduction

The rapid proliferation of AI and data analytics in life sciences has led to significant advancements in research methodologies, clinical workflows, and personalized treatment strategies. AI-driven computational models leverage high-dimensional biological data, accelerating hypothesis generation and enhancing the reproducibility of scientific findings (Collins & Varmus, 2015). Key drivers behind AI’s expanding role in life sciences include increasing computational power, large-scale biomedical datasets, and advancements in deep learning architectures.
The incorporation of AI in life sciences has revolutionized the ability to interpret complex biological systems, simulate drug interactions, and predict disease outcomes with high precision. Moreover, AI-based automation has significantly enhanced laboratory operations, reducing human error and increasing throughput in genomic sequencing and drug screening. The integration of AI into regulatory compliance and pharmacovigilance has further enabled healthcare systems to ensure the safety and efficacy of medical interventions while minimizing delays in approval processes.
This report provides an analytical review of AI applications in drug discovery, precision diagnostics, and genomics, alongside a discussion on the challenges of AI adoption. The introduction contextualizes AI’s historical evolution in biomedical research and its transition from rule-based systems to modern deep learning paradigms. Furthermore, AI’s role in predictive analytics for disease outbreaks, computational biology, and automated laboratory workflows is examined, highlighting its transformative potential across various sectors of life sciences (Topol, 2019).
AI in Drug Discovery and Development
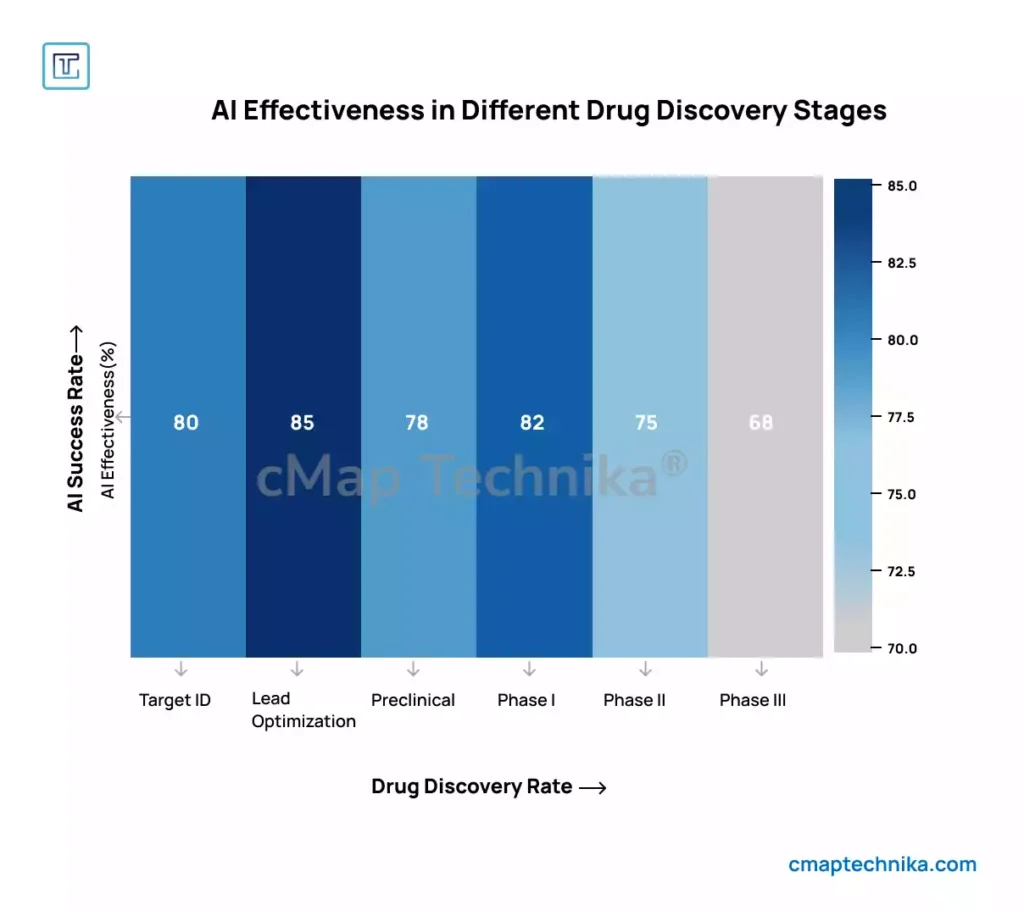
Limitations of Traditional Drug Discovery Pipelines
Traditional drug discovery is characterized by prolonged development cycles, high attrition rates, and escalating R&D costs. The average time from initial drug discovery to market approval exceeds 10 years, with estimated expenditures surpassing $2.6 billion per drug candidate (DiMasi et al., 2016). Failure in late-stage clinical trials remains a major bottleneck, often due to inadequate target validation, off-target toxicity, or suboptimal patient stratification. Conventional in vitro and in vivo screening methods rely on exhaustive experimentation, making the process resource-intensive and inefficient.
Machine Learning in Drug Target Identification and Lead Optimization
AI-powered computational biology integrates multi-omics data, including genomics, transcriptomics, proteomics, and metabolomics, to enhance target discovery. Deep learning algorithms, such as convolutional neural networks (CNNs) and graph neural networks (GNNs), are employed for molecular property prediction, ligand-receptor binding affinity modeling, and de novo drug design (Zhavoronkov et al., 2019). AI-driven generative models, including variational autoencoders (VAEs) and generative adversarial networks (GANs), facilitate the synthesis of novel molecular structures with optimized pharmacokinetic profiles. Transfer learning and reinforcement learning approaches further enhance molecular docking simulations, improving the efficiency of hit-to-lead optimization.
AI-Driven Clinical Trial Optimization
AI has transformed clinical trials by integrating predictive analytics, patient stratification models, and adaptive trial designs. Traditional clinical trials often suffer from high costs, lengthy timelines, and inefficiencies in patient recruitment. AI-driven solutions streamline these processes by leveraging electronic health records (EHRs) and real-world data to identify eligible participants more efficiently.
Machine learning algorithms analyze historical clinical trial data to predict patient eligibility and response rates, leading to more effective participant selection (Esteva et al., 2017). AI also enhances trial monitoring through automated anomaly detection, reducing the risk of protocol deviations and non-compliance. Federated learning frameworks allow AI models to be trained on decentralized datasets, ensuring patient privacy while enabling robust insights.
Furthermore, AI-driven natural language processing (NLP) enables the automatic extraction of critical information from clinical trial documents, reducing administrative burdens and improving regulatory compliance. Bayesian modeling techniques facilitate adaptive trial designs, allowing real-time adjustments based on interim analysis of patient responses. This leads to a more dynamic, efficient, and patient-centric approach to clinical trials.
Case Study: AI in SARS-CoV-2 Drug Discovery
During the COVID-19 pandemic, AI-driven computational methods significantly accelerated antiviral drug discovery (Jumper et al., 2021). DeepMind’s AlphaFold2 revolutionized protein structure prediction, enabling high-accuracy modeling of SARS-CoV-2 spike proteins, which facilitated vaccine and therapeutic development. AI-based molecular docking simulations identified potential inhibitors for viral proteases, expediting lead optimization.
Several pharmaceutical companies, including BenevolentAI and Insilico Medicine, leveraged deep learning models to analyze vast biomedical datasets and identify repurposed drugs for COVID-19 treatment. AI-assisted clinical trial management systems, including IBM Watson and BenevolentAI, streamlined patient recruitment and adaptive protocol adjustments, leading to faster drug approval timelines.
Additionally, AI-driven epidemiological models provided real-time predictions of COVID-19 transmission patterns, enabling public health officials to implement targeted interventions. AI-enhanced contact tracing applications and predictive analytics platforms contributed to the efficient allocation of healthcare resources, further demonstrating AI’s impact in pandemic response efforts.
AI in Precision Medicine and Diagnostics
AI-powered radiomics has significantly advanced medical imaging by enabling automated feature extraction from MRI, CT, and PET scans. Deep learning algorithms such as convolutional neural networks (CNNs) and transformer-based architectures improve diagnostic accuracy and reduce human errors in image interpretation (Esteva et al., 2017). AI-driven segmentation techniques facilitate precise tumor localization, aiding in early cancer detection.
Beyond diagnostics, AI-powered radiomics is revolutionizing treatment planning. AI-based radiotherapy optimization models ensure precise radiation dosage calculations, minimizing damage to healthy tissues while maximizing therapeutic efficacy. AI-enhanced image reconstruction techniques improve resolution in low-dose CT scans, reducing patient radiation exposure.
Additionally, federated learning frameworks allow multiple institutions to collaboratively train AI models on decentralized imaging datasets, overcoming data-sharing barriers while preserving patient privacy. These advancements position AI as an indispensable tool in precision oncology and radiology.
AI-Driven Multi-Omics Integration for Personalized Therapies

AI-driven multi-omics integration enables the convergence of genomics, epigenomics, proteomics, and metabolomics, facilitating precision medicine. Graph neural networks (GNNs) and deep reinforcement learning models analyze multi-modal biological data to uncover novel disease biomarkers and therapeutic targets (Collins & Varmus, 2015).
By leveraging AI, researchers can predict gene-disease associations, identify drug-gene interactions, and optimize treatment regimens based on individual genetic profiles. AI-powered single-cell RNA sequencing analysis provides insights into cellular heterogeneity, aiding in the development of targeted therapies.
Moreover, AI-driven computational pathology integrates multi-omics data with digital histopathology, enabling precise disease subtyping and prognosis prediction. AI-enhanced pharmacogenomics models analyze genetic variations to predict drug responses, reducing adverse drug reactions and optimizing dosing strategies.
Future developments in AI-driven personalized medicine include the integration of quantum computing for high-dimensional data analysis and the use of explainable AI (XAI) models to enhance interpretability in clinical decision-making. These innovations are expected to drive a paradigm shift in individualized healthcare.
Case Study: IBM Watson in Oncology Decision Support
IBM Watson for Oncology integrates NLP and machine learning algorithms to analyze vast repositories of clinical literature and patient records (Ferrucci et al., 2013). The AI system provides oncologists with evidence-based treatment recommendations by cross-referencing genomic alterations, treatment response data, and clinical trial eligibility criteria. Watson’s AI-assisted decision-making reduces diagnostic uncertainty and facilitates personalized treatment planning, demonstrating the utility of AI-driven clinical decision support systems in oncology.
Challenges and Future Directions
Ethical and Regulatory Considerations
The adoption of AI in life sciences necessitates adherence to stringent regulatory frameworks, including FDA’s AI/ML-based Software as a Medical Device (SaMD) guidelines and EMA’s AI governance policies. Bias mitigation strategies, data privacy compliance under GDPR and HIPAA regulations, and explainability in AI-driven diagnostics remain critical challenges (Reddy et al., 2020). The integration of blockchain technology for decentralized patient data governance is proposed as a solution to enhance data security and interoperability.
Conclusion
AI and data analytics are reshaping the life sciences industry by accelerating drug discovery, optimizing clinical trials, enhancing medical imaging, and enabling personalized therapies. The integration of AI with multi-omics data, real-world evidence, and federated learning frameworks is revolutionizing precision medicine.
Despite its transformative potential, AI adoption in life sciences faces challenges related to regulatory compliance, data privacy, and ethical considerations. Future research should focus on developing transparent, interpretable, and unbiased AI models to ensure equitable healthcare outcomes. As AI continues to evolve, its convergence with blockchain, quantum computing, and synthetic biology will further redefine the landscape of biomedical innovation.
References
- Collins, F. S., & Varmus, H. (2015). A new initiative on precision medicine. New England Journal of Medicine, 372(9), 793-795.
- DiMasi, J. A., et al. (2016). Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of Health Economics, 47, 20-33.
- Esteva, A., et al. (2017). Dermatologist-level classification of skin cancer with deep neural networks. Nature, 542(7639), 115-118.
- Ferrucci, D., et al. (2013). Watson: Beyond jeopardy! Artificial Intelligence, 199, 93-105.
- Jumper, J., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature, 596(7873), 583-589.
- Reddy, S., et al. (2020). A governance model for the application of AI in health care. The Lancet Digital Health, 2(9), e447-e449.
Topol, E. J. (2019). High-performance medicine: the convergence of human and artificial intelligence. Nature Medicine, 25(1), 44-56.